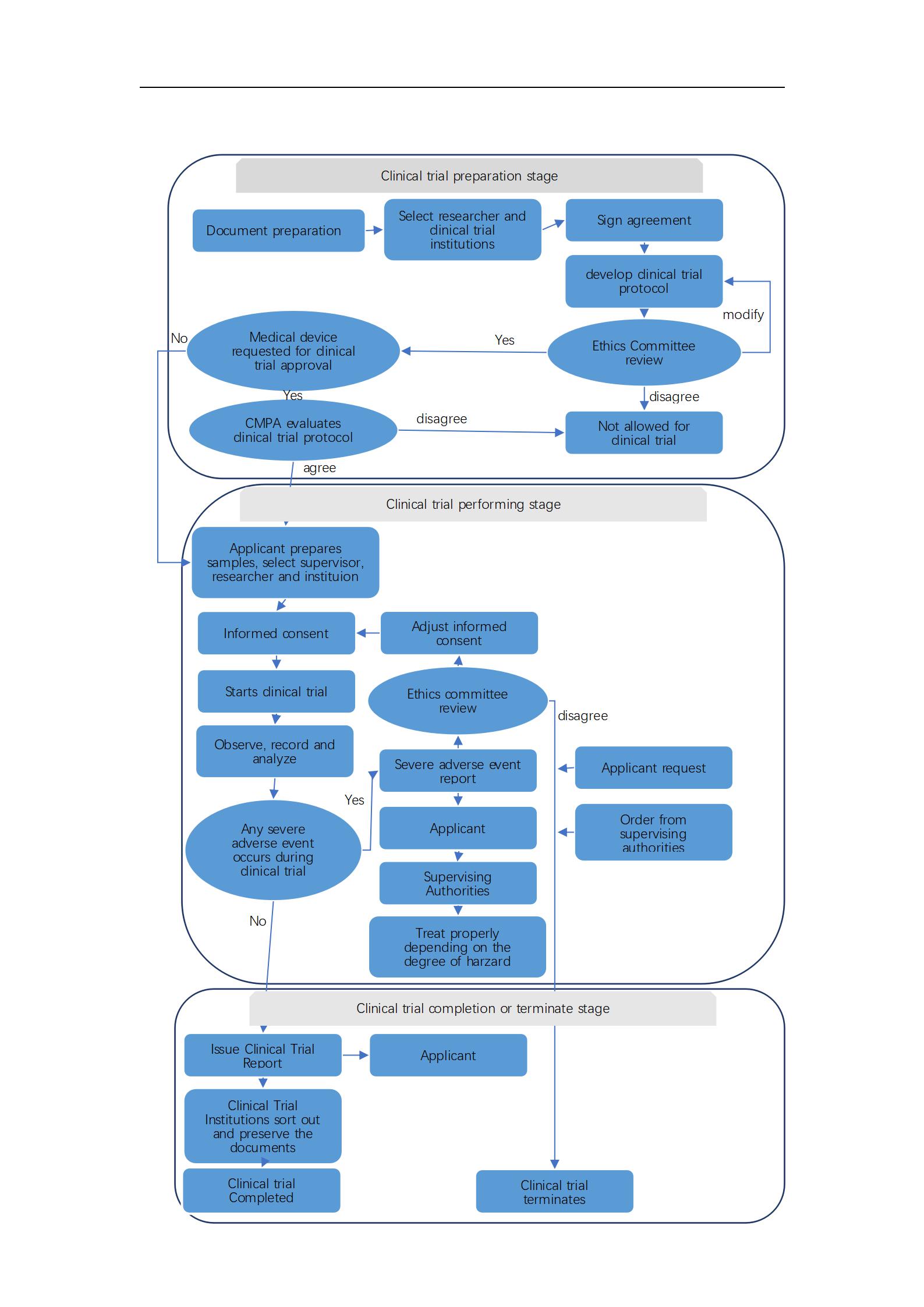
1. Regulations on clinical trial
According to the Supervision and Administration Regulation for Medical Device (by State Council No.650), Record-filing is required for Class I medical device without performing clinical trial, yet submission of clinical appraisal is required. For Class II and Class III medical device, clinical trial is required (except for those exempt from clinical trial ). For those exempt from clinical trial, clinical appraisal must be provided during submission of registration files.
2. Service
2.1 To produce qualified product samples for clinical trial in compliance with Quality Management System.
2.2 To provide service of selecting clinical trial center and daily communication.
2.3 To provide service of drafting clinical trial protocol, CRF form and consultation.
2.4 To submit clinical trial approval certificate, record-filing, adverse event report.
2.5 To conduct analysis whether the product is exempt from clinical trial.
3. Procedure