Service And Cooperation
Medical Device Import Registration/Record-Filing
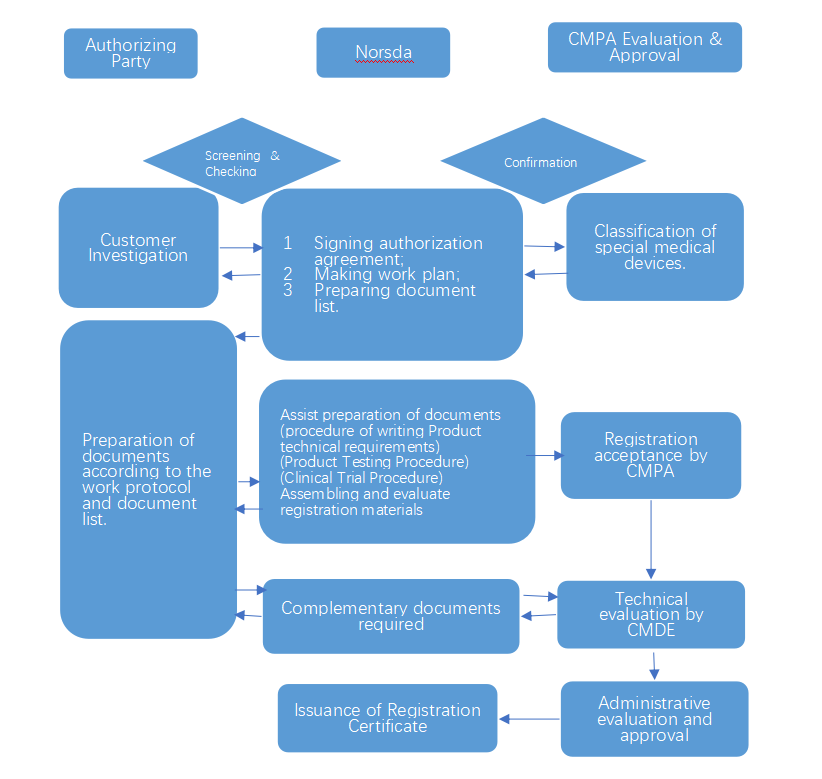
Medical Device Registration
1. Registration Application and Evaluation Process
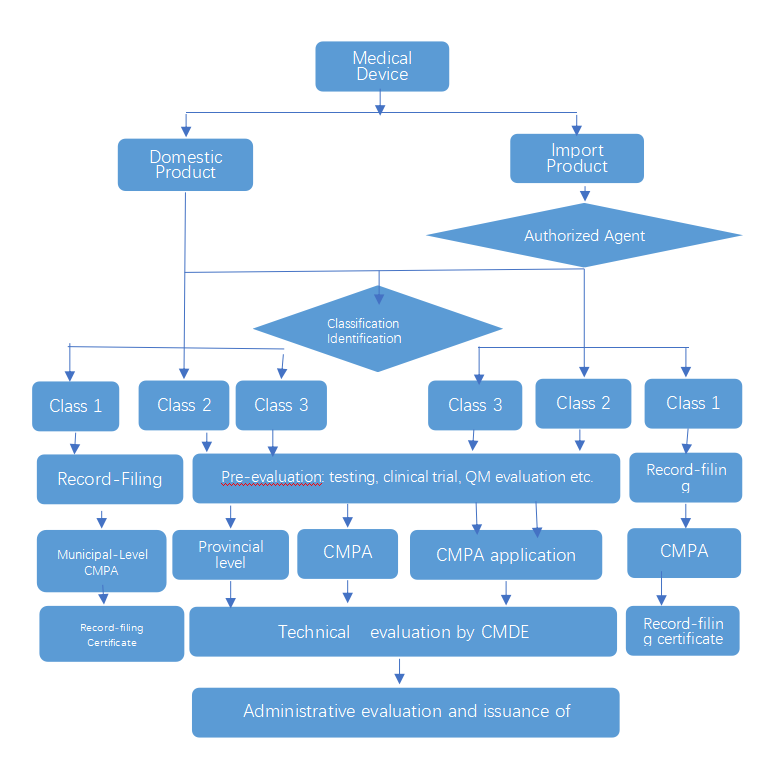
2.Medical device registration service process
Medical Device Record-Filing
Norsda is ready to provide the following service in accordance with regulations of Class 1 medical device issued by NMPA.
1. To draft product technical specifications, instruction and label;
2. To provide Product Property Testing Service;
3. To draft Product Clinical Appraisal;
4. To draft Product Risk Analysis;
5. To draft product technical procedure;
6. To draft Quality Management Brochure and Procedure documents.
7. To Follow up with record-filing process
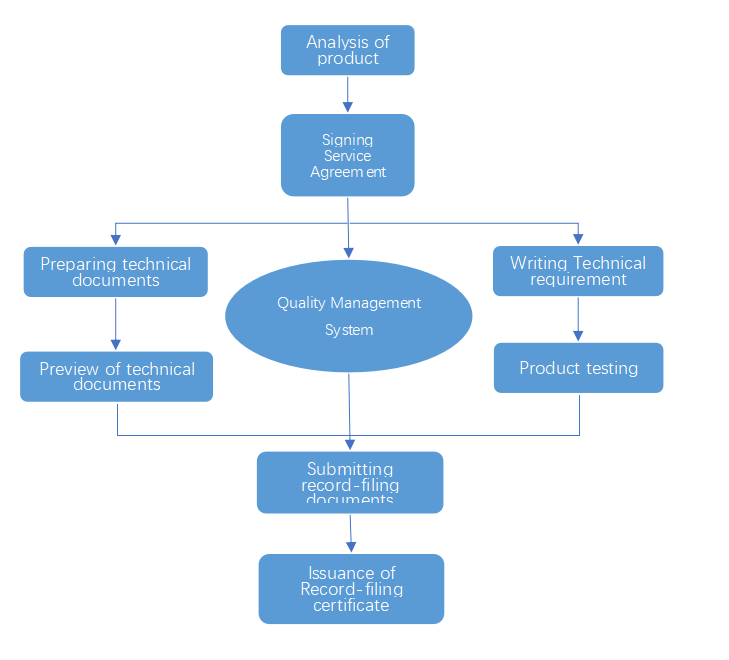
The service flow chart: